When Work Is Done On A System
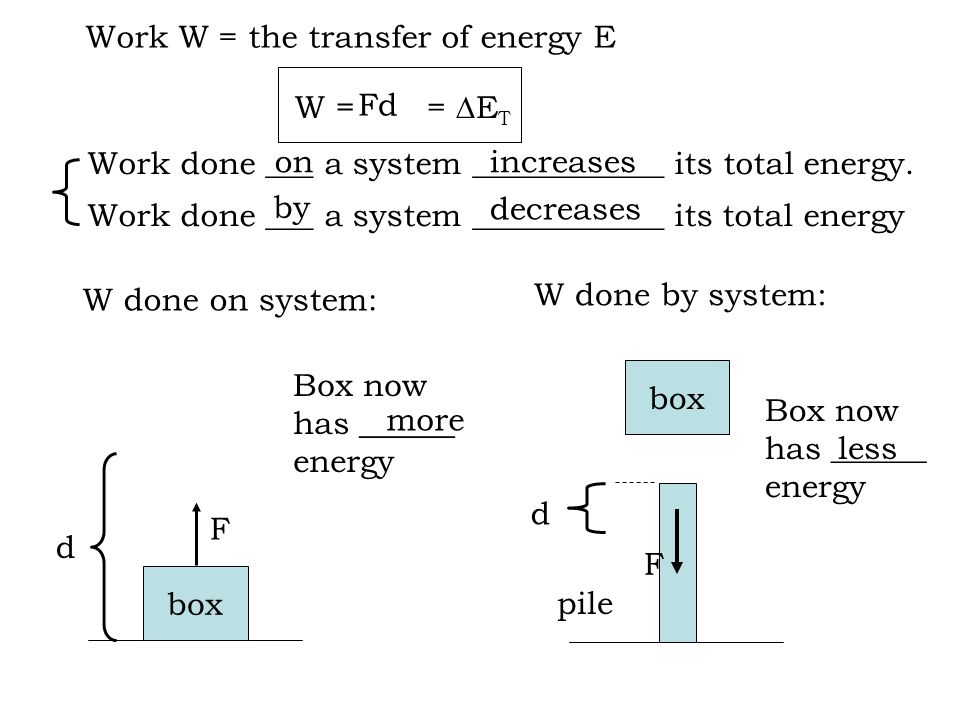
When work is done on a system. If work is done on the system energy added to the system the work is negative. Work is the measure of energy transfer when a force F moves an object through a distance d. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments.
As work is done on the system therefore dW -300J Again as 70 cal Of heat is extracted from the system. The work in turn increases the internal energy of the system. Where m is the mass of the system.
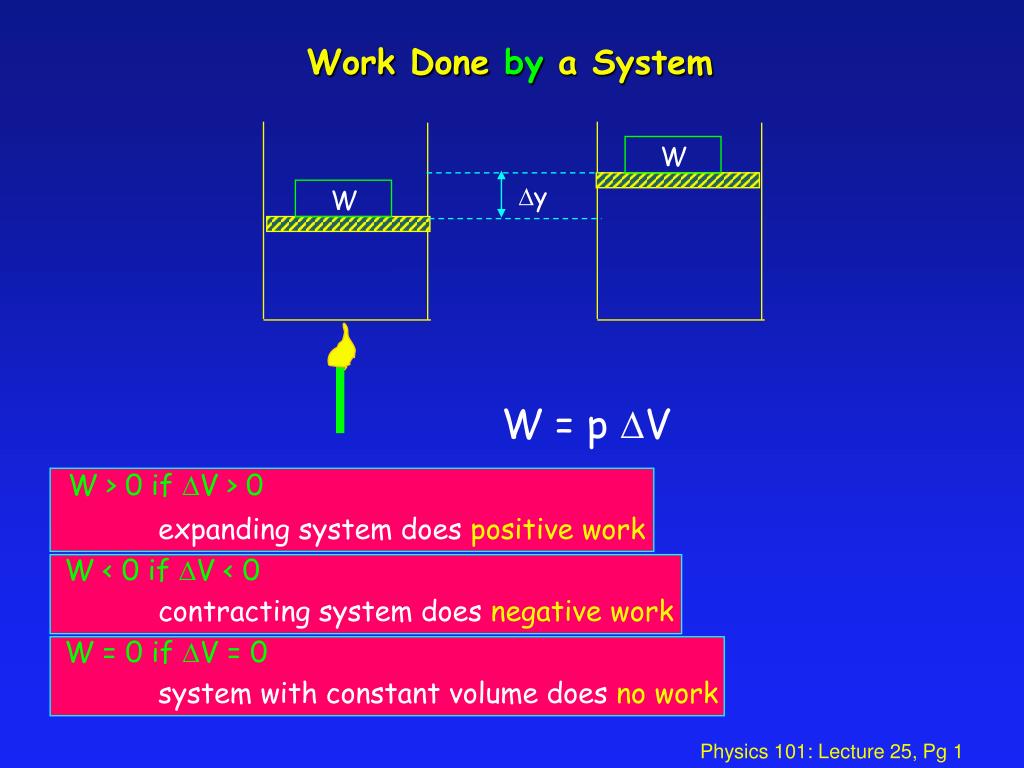
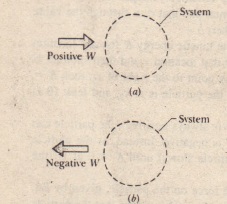
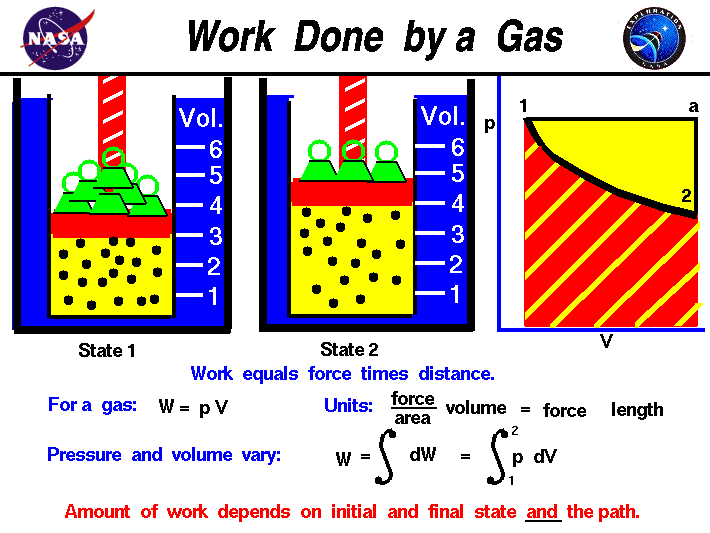
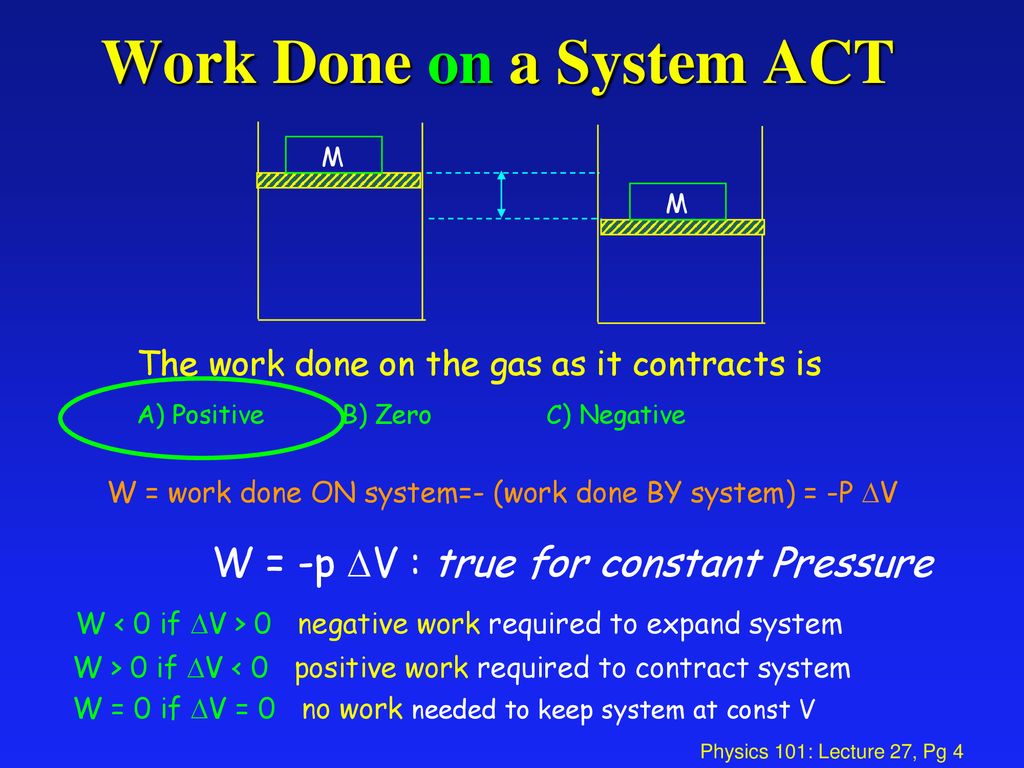
If the total work is a negative value that is in case of expansion of gas we say that the work is done by the system and. If system volume expands against a force work is done by the system. If the initial internal energy of the gas was.
Thermodynamics is a branch of physics which deals with the energy and work of a system. Work Done on System or By System. So when work is.
Work is zero if applied force is zero W0 if F0. A system does work when it expands against an external pressure which means that the pressure of the gas is greater than the external pressure. There are two types of things in the science world - the system and the surroundings.
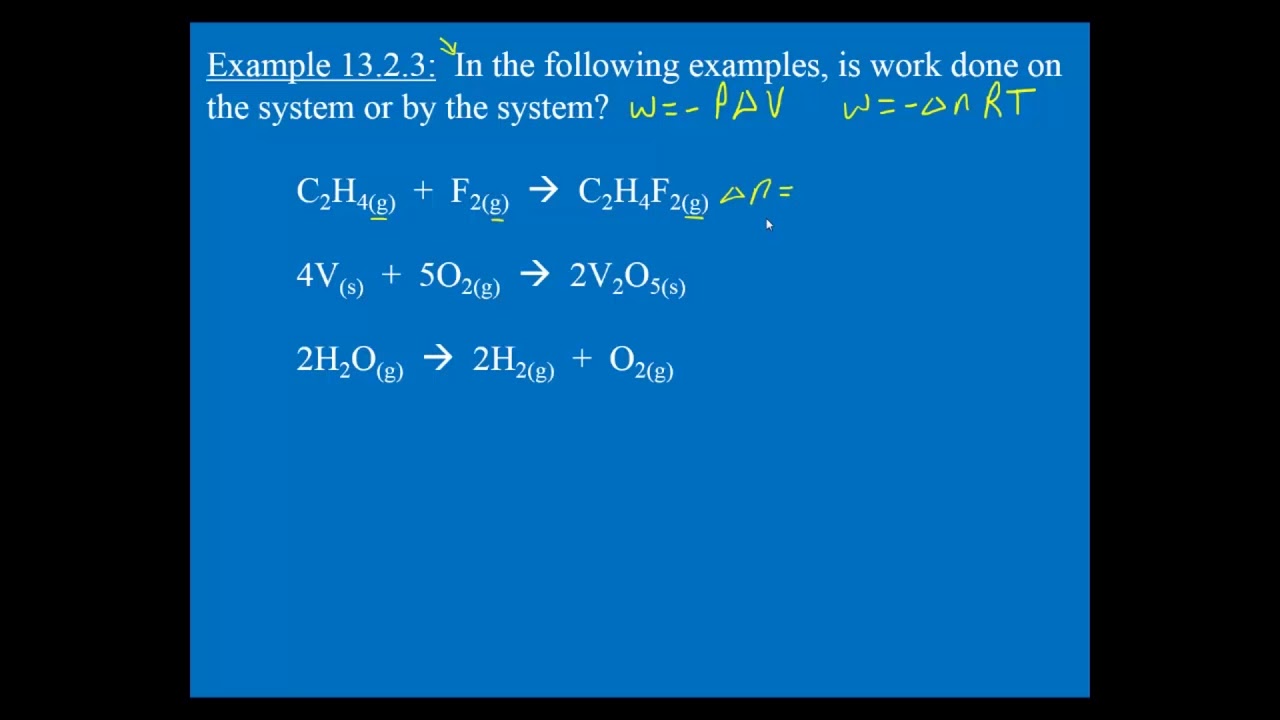
The mathematical relationships between total. Heat is the energy transferred between two objects or two parts of a system because of a temperature difference. Although work done on a system can be any type of work there are a couple of things you should keep in mind with this question.
Throwing a ball means a hand applies a force as an arm swings forward. In general work is defined for mechanical systems as the action of a force on an object through a distance.
For example if a person tries to push a wall he is applying force yet the wall does not move so the displacement of.
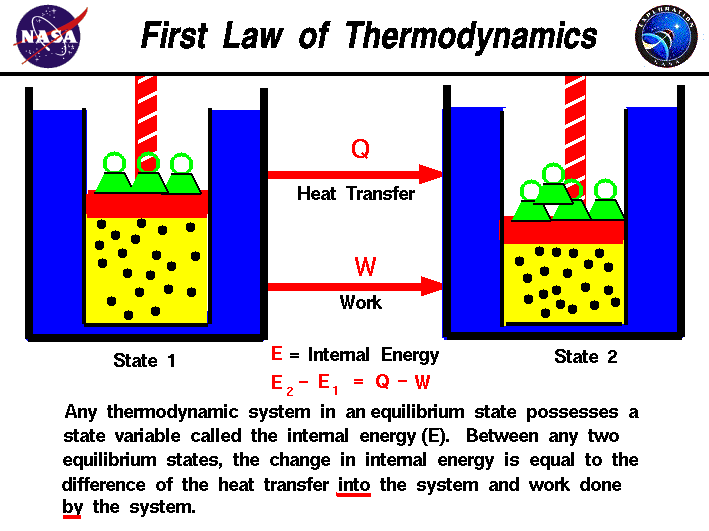
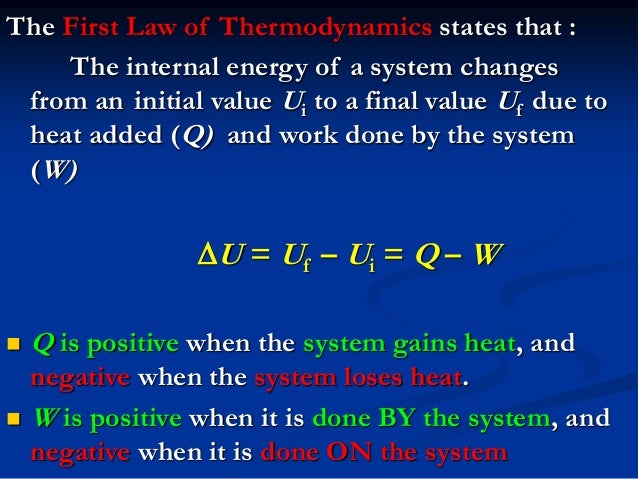
These systems are classified accordingly as you have already studied in section 612. Work Done on System or By System. The first law is simply a conservation of energy equation. W F. Although work done on a system can be any type of work there are a couple of things you should keep in mind with this question. What about the. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. By the piston the force is supplied by the power stroke of a different cylinder ON the exhaust gas as it is pushed out of the cylinder. If the initial internal energy of the gas was.
Heat is the energy transferred between two objects or two parts of a system because of a temperature difference. In thermodynamics work is defined as -p_exDelta V for an ideal gas. Internal energy of a thermodynamic system is its total mechanical energy. Zero work is done when the displacement of a body is zero or perpendicular θ900cosθ0 to the direction of force applied then work done is zero. Work is zero if applied force is zero W0 if F0. If your frame of reference is system then the work done on the system W is positive and the heat that is added to the system is also positive which means the change in internal energy is also positive by first law of thermodynamics which means that there is an increase in temperature. There are two types of things in the science world - the system and the surroundings.



























Post a Comment for "When Work Is Done On A System"